By using this website, you agree to our Privacy Policy
×
What is Rubber?

Raw Rubber is a Natural Product
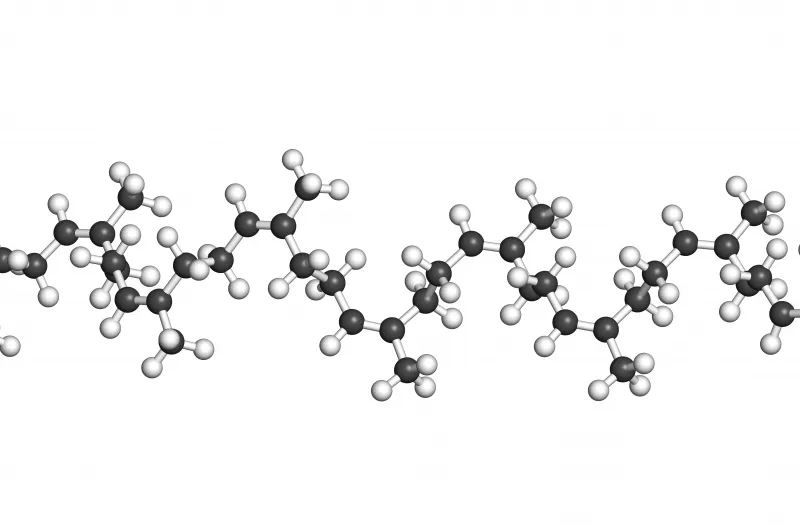
Raw rubber is a natural product made from plants; it is made up of molecules that are a very, very long chain of carbon atoms with repeating sets of attached atoms such as hydrogen, chlorine, fluorine or groups such as methyl and acrylonitrile.
The different elements and groups change the properties of the rubber.
All rubbers are polymers.
A Natural Rubber molecule may have between 20,000 and 120,000 Carbon atoms in its chain with attached hydrogen and methyl groups. (C4H5 CH3)n
Raw rubber is stable in a tangled state. The forces between the molecules are weak, so the application of external force can shape them.
However, its elastic nature means it will try to recover its preferred configuration after deformation.


Natural Rubber
Natural rubber or caoutchouc is a colloidal dispersion in an aqueous solution (latex) produced by many plants.
Latex should not be confused with tree sap. It is thought that it acts mainly as a defence against herbivorous insects and slugs. It has a milk-like consistency which coagulates on exposure to air.
It is chemically the cis-isomer of polyisoprene.
Although over 10% of plants produce latex, for example, the dandelion, only the Hevea Brasiliensis tree has proved to be commercially viable, yielding over 99% of the natural rubber latex produced today.
Originating in the Amazon region of South America, courtesy of industrial espionage activity by the British at the end of the 19th Century, natural rubber is now grown widely in tropical climates, particularly in Asia.
Much of South America is seriously affected by leaf blight and can no longer sustain large plantations.
Resources PDFs
We’ll Get Straight Back to You
Explore our other Industries
Speak to One of Our Experts
Contact UsLEARN
INDUSTRIES
PRODUCTS
BRANDS
GET IN TOUCH


- © 2025 Nufox. All rights reserved |
- Terms & Conditions |
- Privacy Policy |
- Download ISO Certificate | Web Design MadeByShape